Quality Assurance System
MicroBiopharm Japan Co., Ltd. (hereinafter referred to as "MBJ") considers the quality of pharmaceuticals and other products first, builds an advanced quality assurance system and each individual makes daily efforts to deliver our products which contribute to the health of people (Our Quality Policy).
- MBJ maintains marketing approval of pharmaceuticals at headquarter and licenses for manufacturing of pharmaceuticals at each plant. MBJ also has licenses and registries for each product, such as feed and food additives, and conducts production activities in compliance with the laws and regulations.
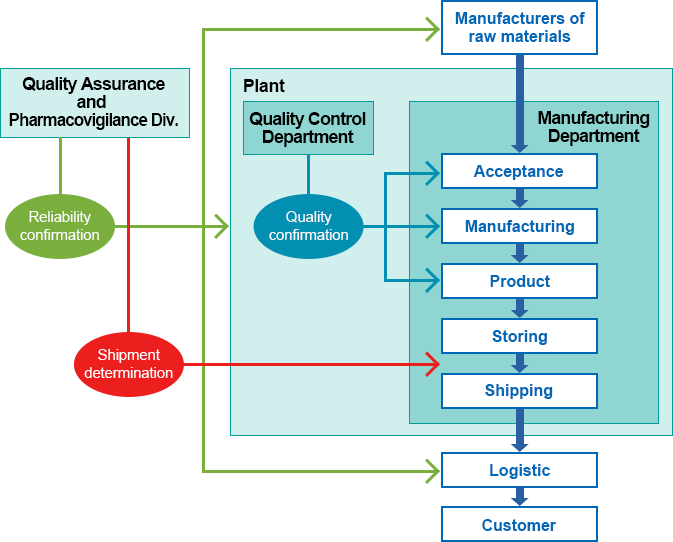
- MBJ has a Quality Assurance and Pharmacovigilance Division (QA) independently of the R&D, marketing, and production sections to ensure the objectivity of quality-related judgments while QA staffs engaging in quality assurance activities including product releasing decisions are present at each manufacturing site.
- The standards, procedures and records have been well developed and documented in compliance with Good Manufacturing Practice (GMP), and our qualified employees who have received education and training engage in manufacturing or quality controls in compliance with these standards and procedures.
- MBJ has established a consistent quality assurance system throughout the lifecycle of our products from the development stage to commercial production.
- MBJ maintains regulatory filings such as Drug master files in Japan, the United States, Europe, China, etc., and production and quality control activities are carried out in conformity with these registrations.
- MBJ has accumulated compliance results with inspections by the U.S. FDA and domestic regulatory authorities. In addition, MBJ actively receives audits by domestic and overseas customers and each observation from the customers is made into good opportunity for improvement of our quality assurance system.
- MBJ always makes improvements with our continuous efforts by paying much attention to trends in regulations such as GMP ordinance, ICH guidelines, and PIC/S guidelines to stay compliant with up-to-date requirements.